More ethanol, fewer emissions, land with multiple purposes—there are several advantages of using renewable electricity to increase the carbon efficiency of biorefineries.
October 18, 2023Dr. Zia Abdullah

Dr. Zia Abdullah is laboratory program manager for the National Renewable Energy Laboratory’s (NREL’s) Bioenergy Technologies Office program.
Zia has extensive experience and accomplishments in thermochemically and biochemically converting biomass to fuels and chemicals. His experience includes more than 25 years of industrial research and development in biomass conversion, as well as problem solving, new product development, business development, and project management.
Prior to joining NREL, Zia was chief technology officer at Versa Renewables LLC, where he developed and commercialized biomass pyrolysis and biopolyol technologies. Before Versa, Zia served as one of the Battelle Memorial Institute’s 11 fellows, leading the development of technology and commercial application for fuels, chemicals, and materials from the thermochemical and biochemical conversion of biomass. Prior to working at Battelle, Zia was a research advisor (fellow) at Weyerhaeuser Company, where he worked on challenges related to manufacturing and biomass valorization.
Meet our other bloggers ►
Return to Bioprose blog ►
More ethanol, fewer emissions, land with multiple purposes—there are several advantages of using renewable electricity to increase the carbon efficiency of biorefineries.

If you think an “electrofuel” sounds like something from a comic book, you’d be forgiven. Made from carbon dioxide (CO2) from industrial waste gases or directly from the air, e-fuels defy conventional wisdom on how liquid fuels are made. Although e-fuels may be technological marvels, they don’t come from another planet. They come from America’s corn belt, or at least they soon could according to new analysis from the National Renewable Energy Laboratory (NREL).
The Technology Beneath the Cape: Carbon Dioxide Electrolyzers
Every hour, a typical 90-million-gallon ethanol plant produces around 27 tonnes of CO2, a natural byproduct of fermentation. These waste streams of CO2 are highly concentrated—almost pure CO2—which makes them a choice ingredient—or feedstock—for making e-fuels by outfitting refineries with electrolyzers. When given an electrical charge, electrolyzers catalyze chemical reactions that split and reconfigure CO2—a stable molecule—into compounds easier tO upgrade into energy-dense fuels and chemicals. In this way, electrolyzers can turn CO2 that would have been released into the atmosphere into valuable chemicals instead. Doing so could not only greatly lower the carbon intensity of ethanol— already with 44%–52% lower greenhouse gas (GHG) emissions than gasoline—but also boost the fuel output of existing facilities.

By how much? Ongoing research from the CO2 Reduction and Upgrading for e-Fuels Consortium (CO2RUe), funded by the U.S. Department of Energy’s Bioenergy Technologies Office (BETO), is demonstrating how specialized microbes can metabolize the products made from CO2 electrolysis into ethanol. That research shows an average biorefinery outfitted with electrolyzers and bioreactors could produce as much as 41 million more gallons of ethanol every year.
Think about the potential at a given facility: CO2 could go from a waste product to the basic ingredient for making up to 50% more ethanol —with a carbon footprint even smaller than today’s corn ethanol. All that’s left is finding an abundant new source of renewable electricity to power the electrolyzers.
When Making Ethanol is a Breeze
Among the windiest parts of the country, corn belt states have long been leaders in wind energy capacity, and are positioned to wield that affordable and abundant resource to make e-fuels. According to NREL analysis, there is ample space for wind turbines in existing corn fields to convert all CO2 from fermentation back into ethanol.
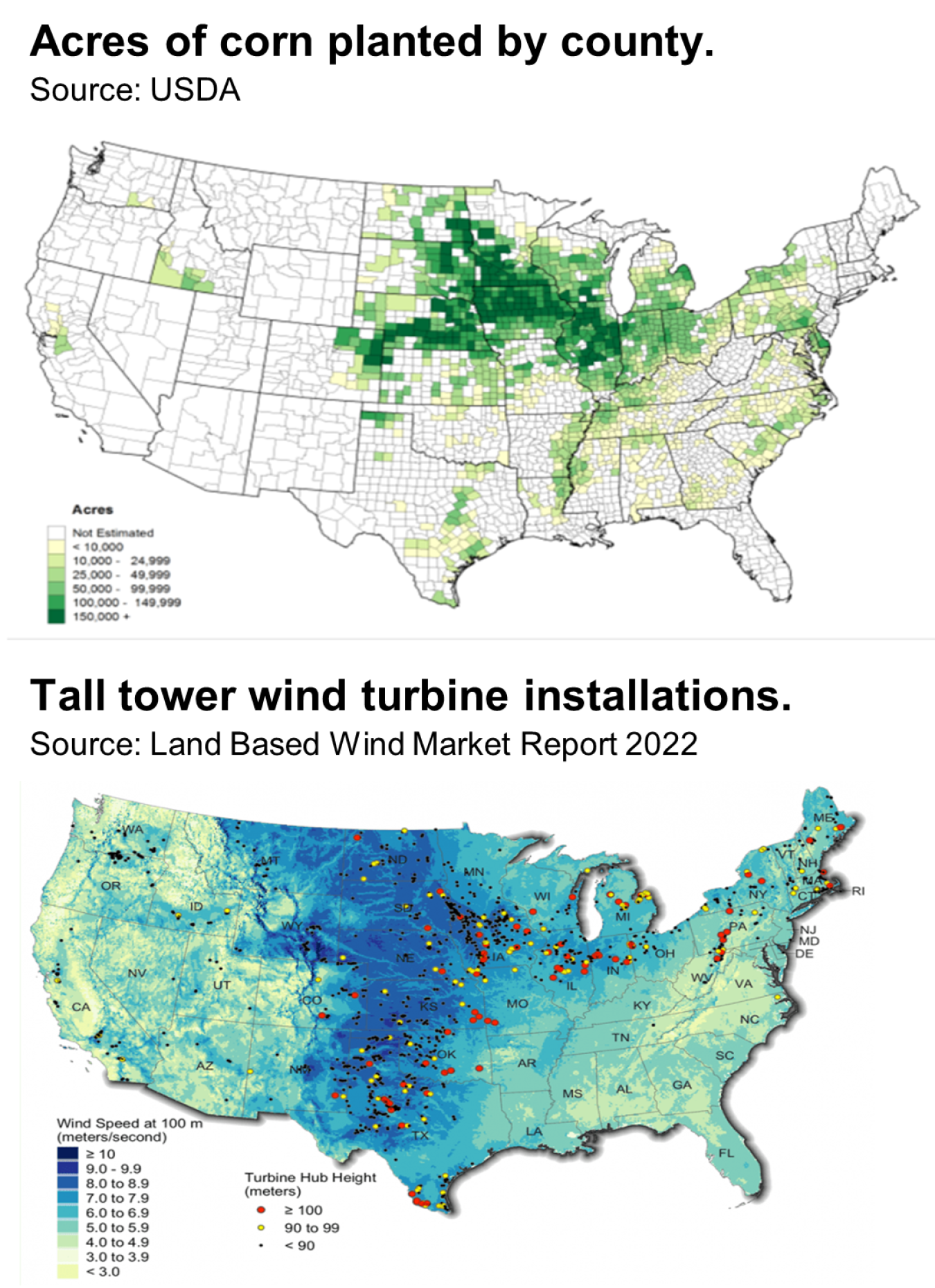
Here’s how the math works for a single, first-generation ethanol plant. It would require 40 megawatts of renewable electricity to power enough electrolyzers to convert approximately half of the hourly output of CO2 into carbon monoxide (CO). Another 165 megawatts is needed to produce clean hydrogen for biologically transforming that CO into more ethanol.
Running at 35% capacity, approximately 200 wind turbines could cover this new demand, and there is ample land to install those turbines in existing corn fields without impacting normal farm operations. In fact, those turbines could be co-located on the approximately 160,000 acres already used to supply a typical ethanol plant.
Reviewing the larger corn ethanol landscape puts these numbers into context. With nearly 200 ethanol plants across the country, countless acres of corn fields, and rapidly falling wind energy costs, midwestern e-fuels could be set to revolutionize the ethanol industry.
Not a Bird, Not a Plane—It’s Jet Fuel Made From CO2
E-fuels are exactly the kind of innovation—call it superpower—we need to meet robust goals to decarbonize transportation, the single largest source of carbon emissions in the United States. Through the Sustainable Aviation Fuel (SAF) Grand Challenge, the federal government aims to boost the production of low-carbon SAF to 35 billion every year by 2050. That’s enough sustainable fuel to cover all anticipated future demand.
Harnessing feedstocks like CO2 alongside the nation’s abundant renewable carbon resources is critical for meeting these bold fuel targets. As BETO-funded researchers have demonstrated, emerging alcohol-to-jet technologies are capable of converting ethanol (whether made from corn starch, corn stover, or waste CO2 from fermentation) into high-performance SAF to meet this demand.
Of course, more targeted research and analysis is essential to improve the readiness of these and other e-fuel processes. Along with technology development, we need innovative business models to seamlessly coordinate transactions between ethanol refineries and wind energy providers. That way, ethanol refineries can focus on what they do best: produce ethanol. Through CO2RUe and other BETO-funded research, NREL and partner institutions are working tirelessly to identify and overcome key barriers to these promising new technologies.
As technologies mature and demand grows for low-carbon SAF from all corners of the country, this NREL analysis raises an exciting and provocative question. 44 million metric tons of pure CO2 from ethanol plants every year; ample wind resources; robust ethanol supply chains; a network of existing biorefinery infrastructure—could these be the underpinnings of a heroic SAF revolution?
CO2RUe, a collaboration of five DOE national laboratories, Argonne National Laboratory, Lawrence Berkeley National Laboratory, Lawrence Livermore National Laboratory, Oak Ridge National Laboratory, and NREL, develops and derisks advanced technologies that use renewable electricity to convert carbon dioxide (CO2) into valuable e-fuels and commodity chemicals. The consortium is funded by DOE’s Bioenergy Technologies Office under its Conversion Technologies subprogram.
Dr. Zia Abdullah is laboratory program manager for the National Renewable Energy Laboratory’s (NREL’s) Bioenergy Technologies Office program.

